All About Innovate Research
Innovate Research is an Indian contract research organization that provides services in clinical research, regulatory affairs, quality assurance and data management.
Industries we Serve
- Pharmaceuticals (Small Molecules and Large Molecules)
- Biologicals/ Biotechnology
- Medical Devices /Medtech
- Food & Beverages
- Nutraceuticals/Herbals/FMCG
- Cosmeceuticals & Cosmetics
Scientific Areas Covering
- Clinical Research Phase I-IV
- RWE/HEOR/PMOS
- Data Management
- IWRS
- Regulatory Affairs & Support
- Quality Assurance
- Biostatistics
- Medical Writing
- Project Management
- Clinical Monitoring
- Site Management
- Study Feasibility
- Biospecimen Collection

Regulatory Agencies We Cater To
Fulfilling needs of every industry







Quality Services With Modern Facilities

Study Feasibility
We identify the best sites through in-depth research and feasibility to determine the best fit for your project. We are quick and responsive, and able to navigate with finesse.

Medical Writing
Innovate’s highly experienced team of medical writers work on a variety of projects from initial protocol development to clinical study reports and final regulatory documentation.

Regulatory Support
Our services range from a simple advisory service consulting to full IND, NDA and IDE preparation and submission, including submissions in electronic Common Technical Document (eCTD) format.

Clinical Monitoring
Our clinical monitoring teams are built to deliver fast, efficient investigator site support and data oversight for your study. Innovate requires our CRAs to demonstrate proficiency in site monitoring before allocated to a study.

Project Management
We provide full service project management support for Phase I through Phase IV trials in major therapeutic areas. We use our skills through formal training and hands on experience managing pharmaceutical and device trials.

Biostatistics Services
Our team of biostatistician and statistical programmers provide a full range of biostatistical services including sample size calculations, randomization schedule generation, blinding and unblinding procedures…

Biospecimen Collection
With a network of hundreds sites across India we are the chosen procurement partner for several research labs, diagnostic, medical device and pharmaceutical companies as well as manufacturers.

Insourcing & Outsourcing
Our team of recruiters have sound scientific background and knowledge along with human resource management skills and they are specialized in scientific and functional candidate identification and recruitment.

Data Management
Our clinical data management processes, expertise and technology are developed to make each step of your clinical trial data management process more accurate and efficient for Phase I-IV in wide range of therapeutic areas.

IWRS services
Innovate provides in-house developed IWRS solutions, or Interactive Web Response Management System ‘PageOne’, as part of its eClinical portfolio.

Site Management
Innovate Research’s SMO team helps to cease the gap between sites and sponsor and/or CRO in accelerating patient enrollment and effectively managing the trial from start to finish.

RWE/HEOR
Real world data (RWD) and Real World Evidence (RWE) are playing an increasing role in health care
We can gather RWE through claims and medical record review and communicate results to support value demonstration for life science industry clients.

Your Trusted Partner from First-in-Human to Post-Marketing Success
Your journey from new molecule to new medicine starts with appropriate subjects, world class facilities, and more timely results. Innovate Research provides comprehensive early phase testing services through our highly experience database of hospital-based clinical units.
When beginning a study, we can rapidly recruit study participants using our in- house database and existing relationships with leading hospitals. A dedicated project team, which includes project management, regulatory, quality and scientific specialists, is assigned to each study in order to develop and execute a program that meets all your requirements. The study is then deployed in a modern clinical facility.
We deliver what is required for you
150
+150
+International & Domestic Clients
22
+22
+Therapeutic Area Expertise
250
+250
+Studies Across All Phases (I/IV)
4
+4
+Phase I on IND Molecules
Certification & Accreditations
See Our Latest Blog
FAQs About Clinical Research Organization
A contract research organization in India provides expert support for the planning, execution, and management of clinical trials, helping sponsors bring new drugs, devices, and therapies to market safely and efficiently.
We manage the full clinical trial process phase I-IV, including protocol development, site selection, patient recruitment, data management, regulatory submissions, and quality assurance, clinical study reports ensuring each study meets global standards.
Our team implements rigorous data management practices, advanced in house electronic data capture systems, and continuous monitoring to guarantee the accuracy, security, and reliability of all clinical trial data.
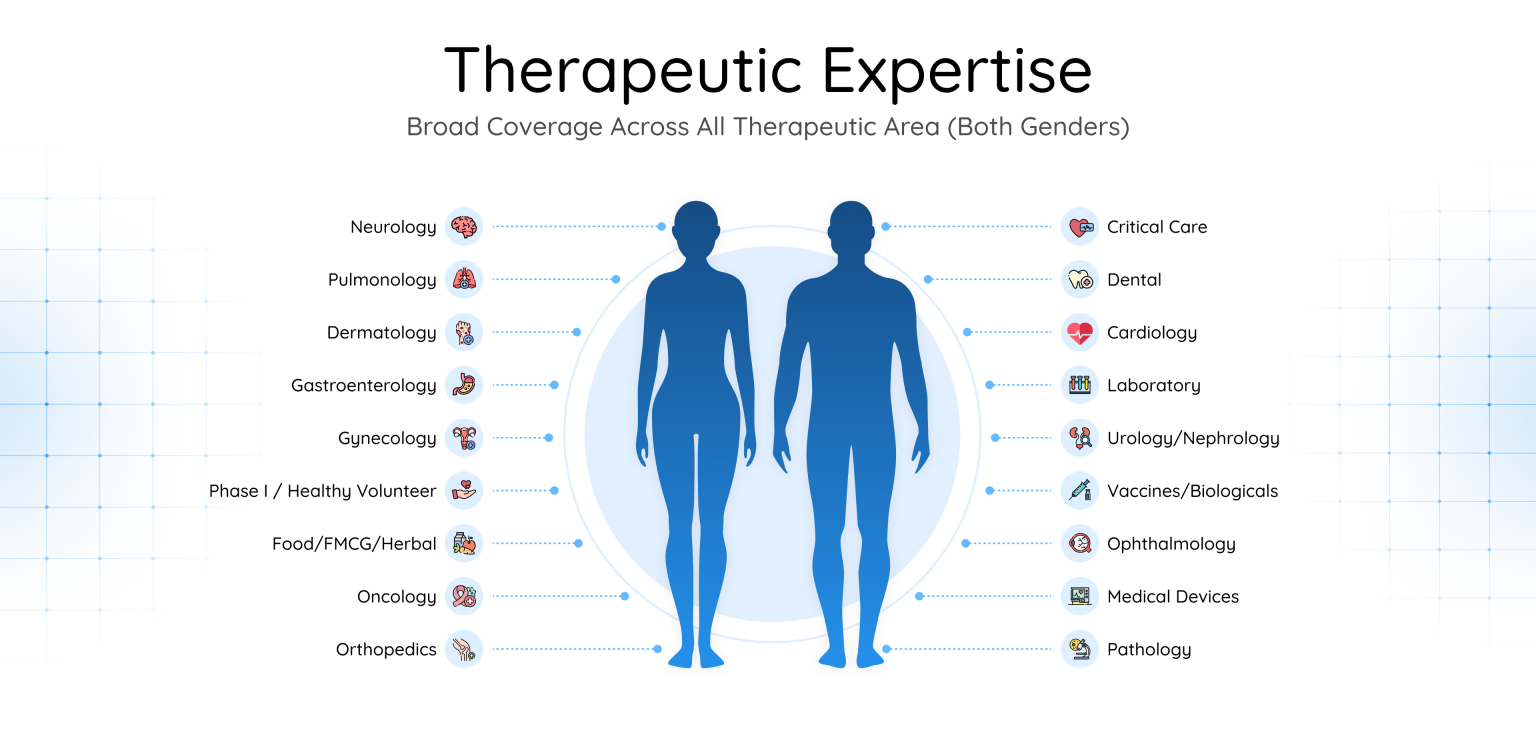
As a leading contract research organization in India, we have expertise across 18+ therapeutic areas, including oncology, cardiology, neurology, endocrinology, infectious diseases, and more.
As one of the best clinical research organization in India, our comprehensive services include clinical trial management (Phases I–IV), regulatory affairs, medical writing, site management, data management, biostatistics and real-world evidence studies/HEOR.