

ClinSoft™
ClinSoft™ was created in-house at Innovate Research without any third-party acquisitions or integrations.
The system is compliant with the FDA requirements (21 CFR Part 11) as well as with GCP and HIPAA.
The system is validated and provide a full audit trail.
ClinSoft™ is convenient to use as it ensures quick start-up and close-out thereby saving your precious time during the study and you can make sure that if there are any mid-study updates or amendments made to a protocol that these changes will be promptly adjusted.
- US FDA 21 CFR Part 11 compliant Validated System
- Electronic Signatures & Records
- Secure, Role Based Access
- Audit Trail
- Intuitive, User Friendly Interface for CRF & Edit Checks Set-up
- Re-usable Frameworks for Item, Panels, Visits & Pages
- Minimum Programming required for Study Set-up
- High System Scalability
- Ideal for Multi-Site, Multi-User Trials
- Study Documents Repository
- Paper Trial Mode
- Double Data Entry (DDE)
- Comparison Reconciliation
- Batch Edit Checks
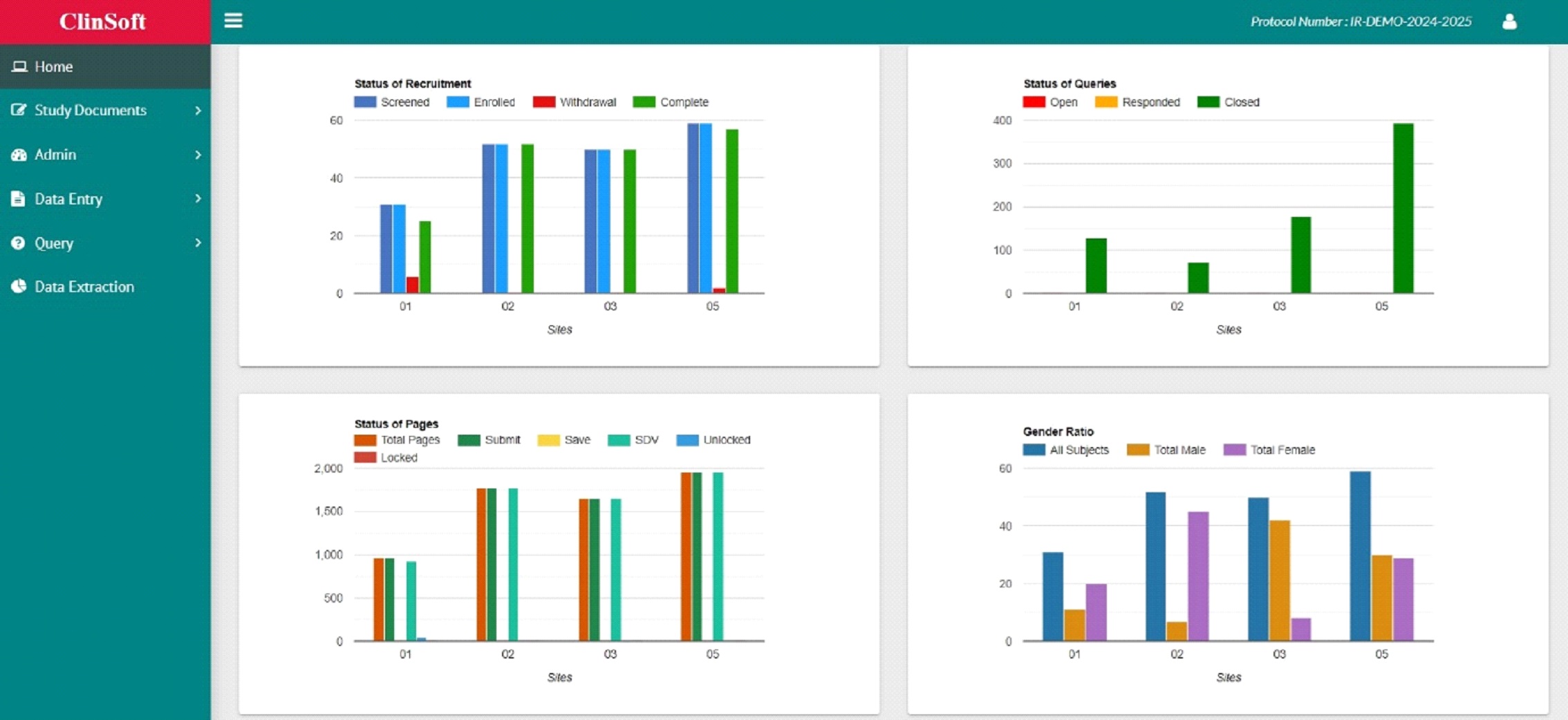
- Customized Reporting & Dashboards
- Project Dashboards
- Adhoc Reports
- Multiple Data Extraction Formats
- CSV, SAS, Others

